Anti-odor properties have been used for years in the apparel industry. Manufacturers apply several different types of textile treatments to inhibit odor and bacterial growth. The major ones are silver-based compounds and the synthetic chemicals like triclosan or triclocarban (all toxic to bacteria). Silver-based technologies made up 9 percent of the anti-odor textile market in 2004, but have since grown to occupy more than a quarter of that category. It is a concern for doctors and environmentalists.
Not until recently, silver used in textile have never been tested for safety when used in consumer goods. Pew Charitable Trust’s Project on Emerging Nanotechnologies found that, even though silver is a natural material, and has been used for medicinal purposes in non-nano form for centuries, silver nanoparticles have been shown to damage lung, liver and kidney function in animals.
In 2014, research by MIT shows that silver nanoparticles, not only can they kill harmful bacteria, can also damage cell DNA. We don’t know exactly how much exposure to silver is harmful to human health, or even how much silver from a fabric treatment on a shirt or shorts makes its way to our skin.
These odor-fighting properties aren’t native to the shirt, they wear off within a few wears and washes, meaning your pricey workout shirt becomes less effective with time. Several environmental groups have called out on the effect of antibacterial clothing on water supplies. Researchers with the Swedish Chemical Agency found that 50 percent of silver, as well as antibacterial chemicals such as triclosan and triclocarban that are commonly used in activewear, rinse out after 10 rounds of washing, ultimately entering the water source. For marine life, silver particles are toxic. As the property becomes more widely used, it could also begin to affect the water supply and food chain.
The easiest way to limit your exposure to silver is to stop wearing clothes without a clear explanation of where their anti-odor and antibacterial properties come from. If you already have silver embedded clothing, the Swiss researchers suggest that showering immediately after your workout will wash silver nanoparticles from your skin and reduce exposure.
Before Neverquit Socks were created, I bought more than 30 pairs of socks from different brands with different material compositions and designs to find the best socks. I have tried silver, bamboo, coconut charcoal, coffee charcoal, and wool socks; but they all had disappointing results in my anti-bacterial and anti-odor expectations. When I was about to give up, I was given a pair of diabetic socks to try out by a friend. I was AMAZED by how it helped to reduce the foot odor from my military issued shoes. When I found out that it was the zinc oxide properties in there I did more research about the material that I have rarely heard of.
In medical applications, zinc oxide is widely used to treat a variety of skin conditions, including dermatitis, itching due to eczema, diaper rash and acne. It is used in products such as baby powder and barrier creams to treat diaper rashes, etc. It also is a component in tape (called “zinc oxide tape”) used by athletes as a bandage to prevent soft tissue damage during workouts. In the apparel application, zinc oxide possesses strong antibacterial properties, especially against odour-causing bacteria, which helps to make our socks stay fresh longer.
Compared to more popular materials (such as silver), zinc oxide is safe and eco-friendly. I have created a table below for a quick reference.
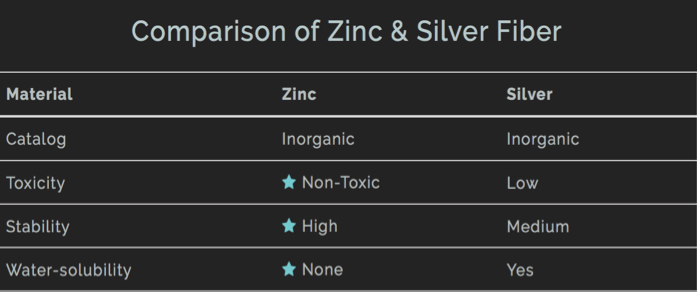
Although zinc is safe and is an essential element to our bodies, “nano zinc oxide” or zinc oxide in nanoparticle form has yet to be proven safe nor harmful. There are some research papers suggest human can overdose zinc oxide when it is in nanoparticle form and damage our health because it can be absorbed via skin surface. If this is your concern, our material is not made of nano zinc oxide. As long as you don’t, uh, eat our cushioned socks, you will be safe from having the zinc oxide particles getting into your system.
I don’t think the zinc oxide material should be limited to only diabetic socks but to all the socks. So by the time I was ready to create the Neverquit Socks, I have already decided to use this material—zinc oxide.
One final fun chemistry fact: zinc oxide can react violently with aluminum and magnesium powders, with chlorinated rubber and linseed oil on heating causing fire and explosion hazard. So please use your socks responsibility.

